![The hybridization of Fe in K4[Fe(CN)6] complex is | Coordination Master Series | Valence Bond Theory - YouTube The hybridization of Fe in K4[Fe(CN)6] complex is | Coordination Master Series | Valence Bond Theory - YouTube](https://i.ytimg.com/vi/E5Dr4k4sbD0/maxresdefault.jpg)
The hybridization of Fe in K4[Fe(CN)6] complex is | Coordination Master Series | Valence Bond Theory - YouTube
![Welcome to Chem Zipper.com......: [Fe(CN)6]3- is weakly paramagnetic while [ Fe(CN)6]4- is diamagnetic, why ? Welcome to Chem Zipper.com......: [Fe(CN)6]3- is weakly paramagnetic while [ Fe(CN)6]4- is diamagnetic, why ?](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEisF7ewBdjRwV9P2QOqyrOrPdt_zrO-MMSNvO-Szjlwoi4icjioowahBpvcDJgYAQQl8Cf2_FbfZHE3MchyoCJfoOULYfJYhOc4iMtBwWEWgf5kpFqRCkoC3qm_8ZG4drbMZrTQdhjlxAM/s1600/1650694296671384-0.png)
Welcome to Chem Zipper.com......: [Fe(CN)6]3- is weakly paramagnetic while [ Fe(CN)6]4- is diamagnetic, why ?
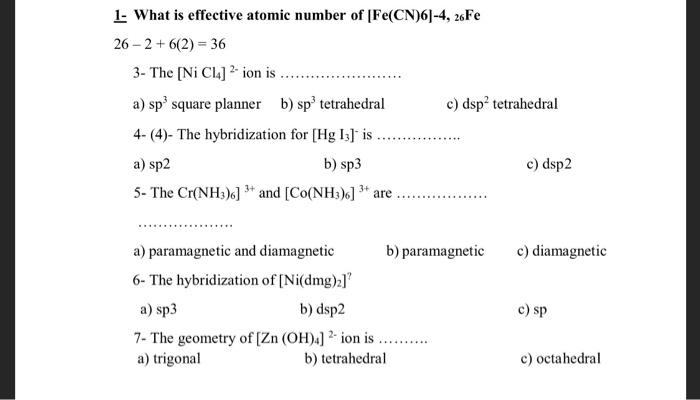
The hybridization and magnetic nature of [Mn(CN)6]^4- and [Fe(CN)6]^3–, respectively are: - Sarthaks eConnect | Largest Online Education Community
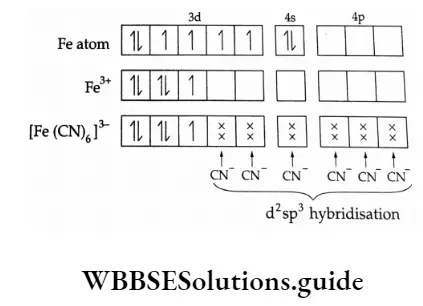
For the complexion of [Fe(CN)6]^3- : (i) Show the hybridization diagrammatically. - Sarthaks eConnect | Largest Online Education Community
Valence Bond Theory For Bonding In Coordination Compounds - Chemistry, Class 12, Coordination Compounds
![a) For the complex [Fe(CN)_(6)]^(3-) , write the hybridisation type, magnetic character and spi... - YouTube a) For the complex [Fe(CN)_(6)]^(3-) , write the hybridisation type, magnetic character and spi... - YouTube](https://i.ytimg.com/vi/ud4xkoAYw-k/maxresdefault.jpg)
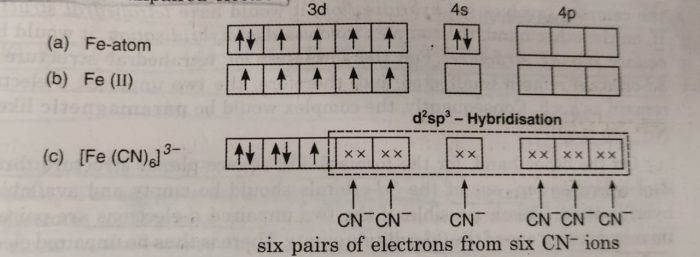
![Writen the hybridization, shape and magnetic character of [Fe(CN)(6)]^ Writen the hybridization, shape and magnetic character of [Fe(CN)(6)]^](https://d10lpgp6xz60nq.cloudfront.net/physics_images/SB_CHM_XII_OD_I_2016_E01_019_S01.png)

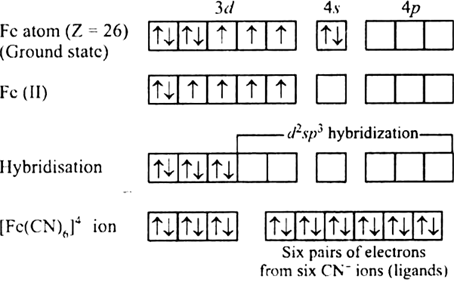
![Solved [Fe(H2O)6]2+,[Fe(CN)6]4− coordination compounds shown | Chegg.com Solved [Fe(H2O)6]2+,[Fe(CN)6]4− coordination compounds shown | Chegg.com](https://media.cheggcdn.com/media/c50/c50287c8-e5c4-44a6-a107-38c183c38ba4/phpPgEih1)
![The hybridization of Fe in K4[Fe(CN)6] complex is: The hybridization of Fe in K4[Fe(CN)6] complex is:](https://d10lpgp6xz60nq.cloudfront.net/physics_images/A2Z_CHM_XII_C09_E01_170_S01.png)
![The hybridization and magnetic nature of [MnCN6]4 and[FeCN6]3 , respectively are: The hybridization and magnetic nature of [MnCN6]4 and[FeCN6]3 , respectively are:](https://byjus-answer-creation.s3.amazonaws.com/uploads/20.PNG_img_upload_solution_2022-06-29%2010:30:57.536595.png)
![3. The hybridization, geometry and magnetic property of [Fe(CN)6]4 are r.. 3. The hybridization, geometry and magnetic property of [Fe(CN)6]4 are r..](https://classroom-images.cdn.askfilo.com/classroom/1653093005879_pqfkqelo_91048.jpg)
![The hybridization and magnetic nature of [MnCN6]4 and[FeCN6]3 , respectively are: The hybridization and magnetic nature of [MnCN6]4 and[FeCN6]3 , respectively are:](https://byjus-answer-creation.s3.amazonaws.com/uploads/7692Chemistry_62bc3161cac8b02f5af0a2d2_c.jpg_img_upload_solution_2022-09-23%2017:22:54.161282.png)
![21. The EAN of Fe in [Fe(CN)6]3 is (1) 26 (3) 38 22. The hybridization o.. 21. The EAN of Fe in [Fe(CN)6]3 is (1) 26 (3) 38 22. The hybridization o..](https://storage.googleapis.com/filo-classroom-notes/thumb_classroom_28163530_F57BH.jpeg)
![Fe(CN)6]4– is diamagnetic while [FeF6]4– i Fe(CN)6]4– is diamagnetic while [FeF6]4– i](https://www.zigya.com/application/uploads/images/chen12070386_571486ba519b3.png)
![Expert Answer] The hybridisation of Fe in K4[Fe(CN)6] is) - Brainly.in Expert Answer] The hybridisation of Fe in K4[Fe(CN)6] is) - Brainly.in](https://hi-static.z-dn.net/files/dbe/9edb7a5f21922ebcbf0ed171935d8835.jpg)
![The hybridization of `Fe` in `K_4[Fe(CN)_6]` complex is: - YouTube The hybridization of `Fe` in `K_4[Fe(CN)_6]` complex is: - YouTube](https://i.ytimg.com/vi/2E532r8xHTg/maxresdefault.jpg)