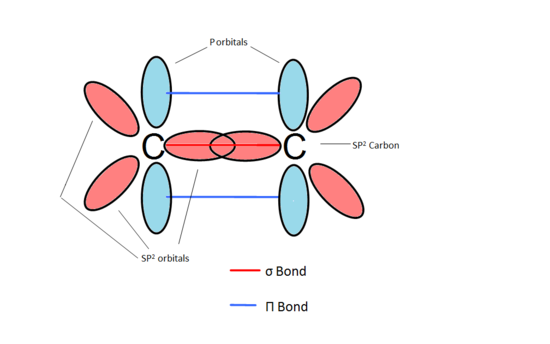
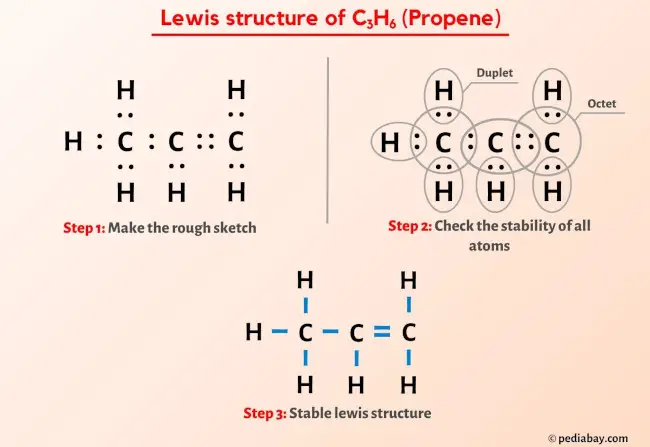
How exactly is this carbon considered sp2 hybridized when it has 3 substituents and a lone pair? : r/OrganicChemistry

Scheme 3. Conformations of propene in the s-p representation (3, 4)a nd... | Download Scientific Diagram
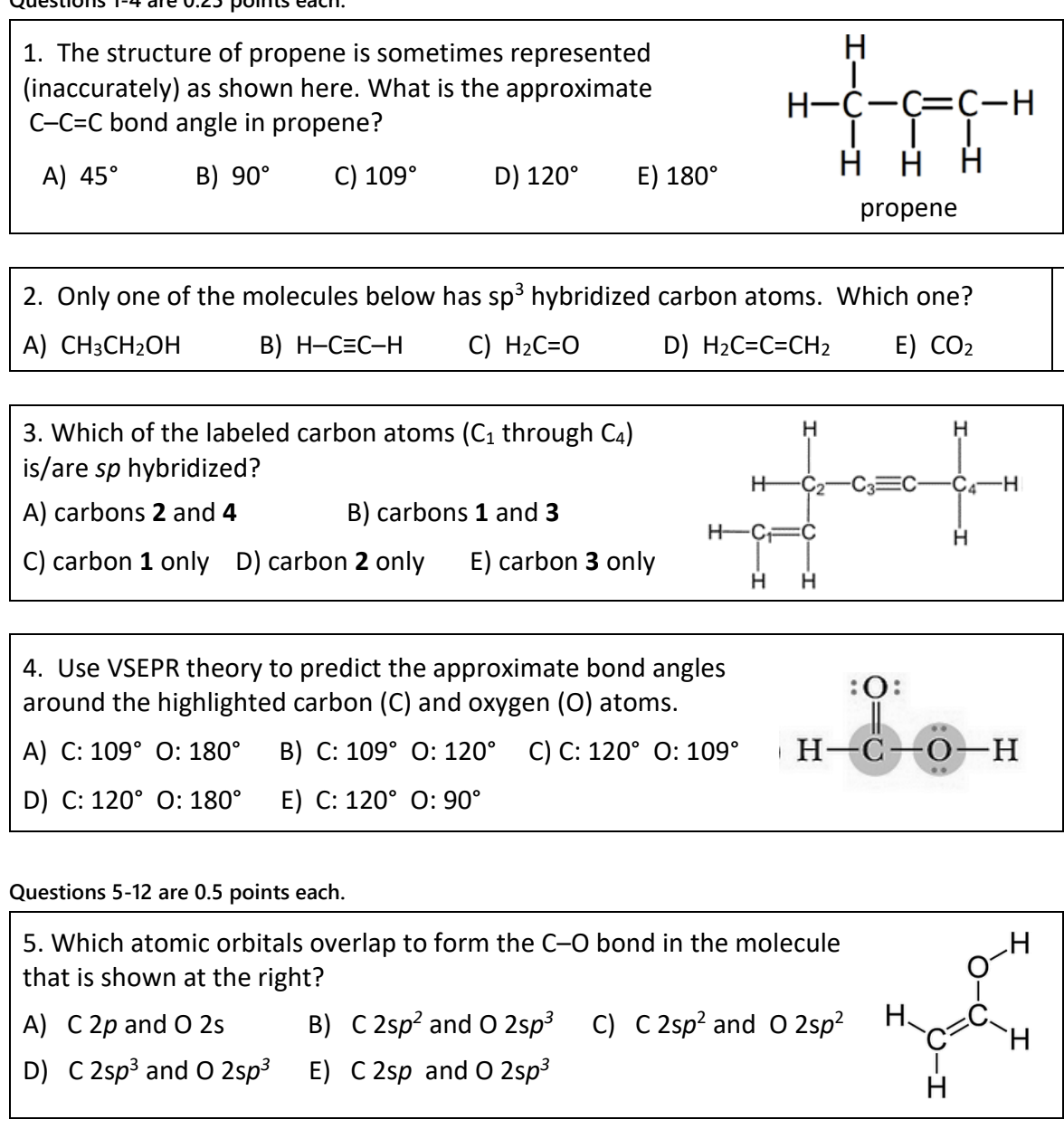
SOLVED: 21 What is the hybridization for each carbon in propene? Carbon on the lefi Carbon in the center Carbon on the right C=C–H H Propene 22 What is the hybridization for
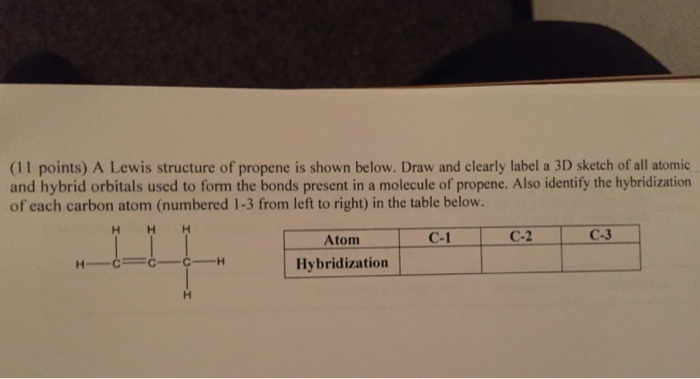
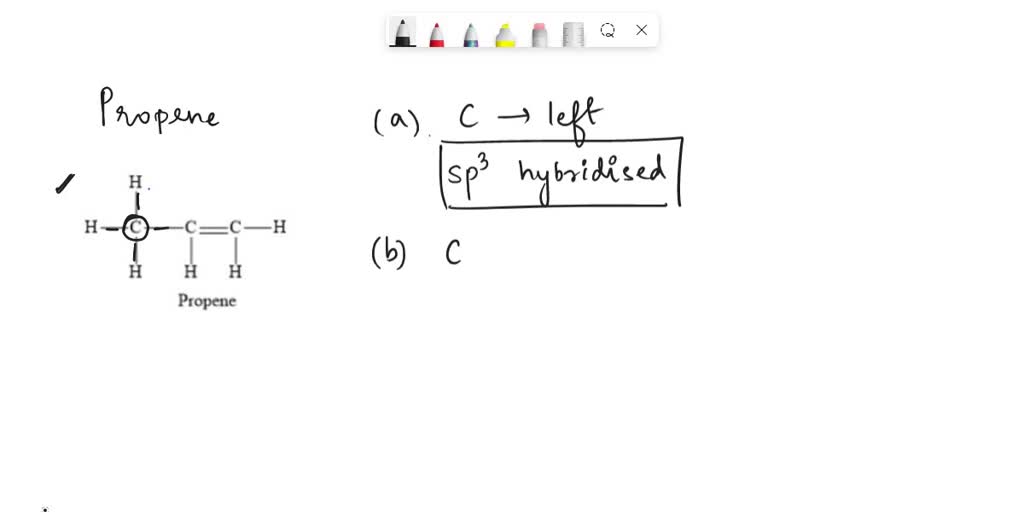
✓ Solved: Draw a line-bond structure for propene, CH3CH=CH2; Indicate the hybridization of the orbitals...
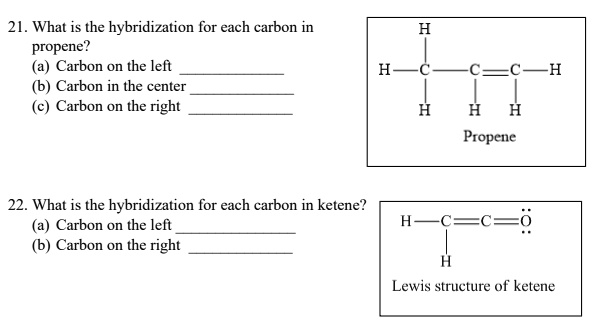
SOLVED: 21 What is the hybridization for each carbon in propene? Carbon on the lefi Carbon in the center Carbon on the right C=C–H H Propene 22 What is the hybridization for

Draw the molecular shape of propene and determine the hybridization of the carbon atoms. Indicate which orbitals overlap with each other to form the bonds. | Homework.Study.com
In the displayed formula of propene, why are the two hydrogens on the right, drawn at an angle? Do I need to replicate these angles when draw the displayed formula of propene? -
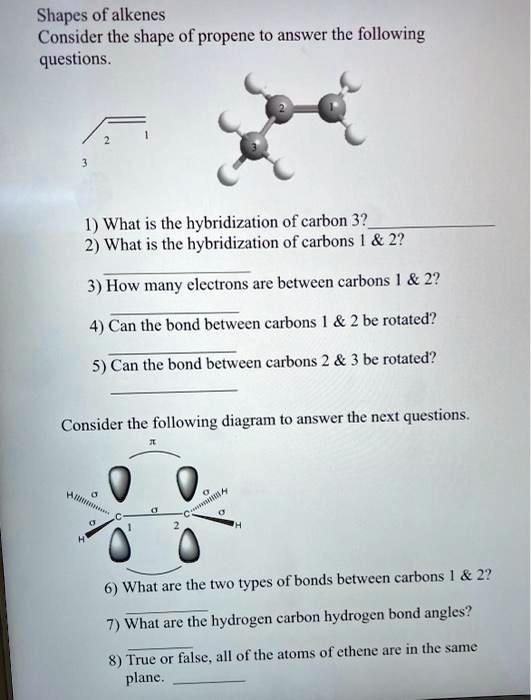
SOLVED: Shapes of alkenes Consider the shape of propene to answer the following questions 1) What is the hybridization of carbon 3? 2) What is the hybridization of carbons | 22 3)
✓ Solved: Draw a line-bond structure for propene, CH3CH=CH2; Indicate the hybridization of the orbitals...
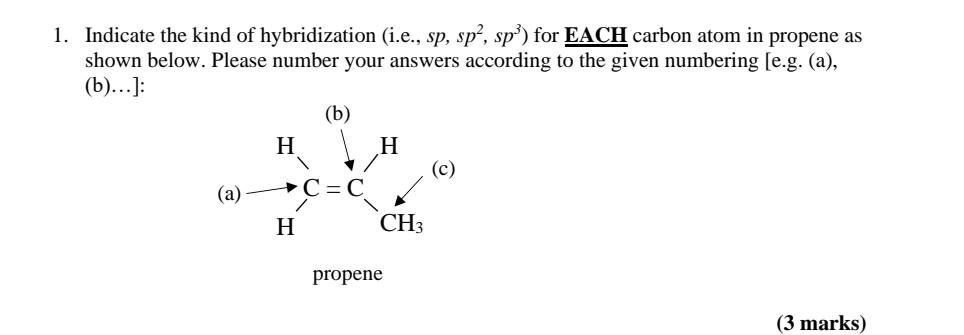
How to draw a line-bond structure for propene, CH3CH≡CH2? What is the hybridization of the orbitals on each carbon - Quora
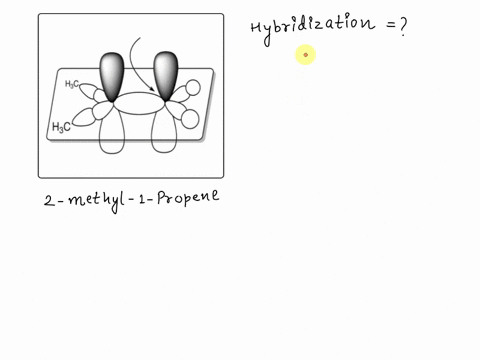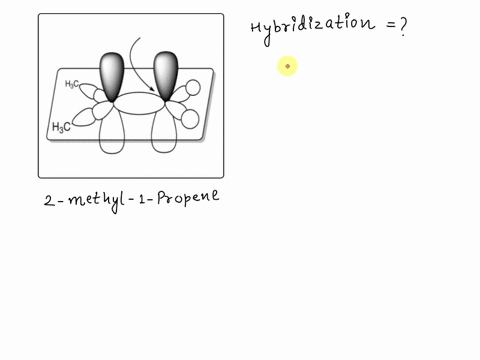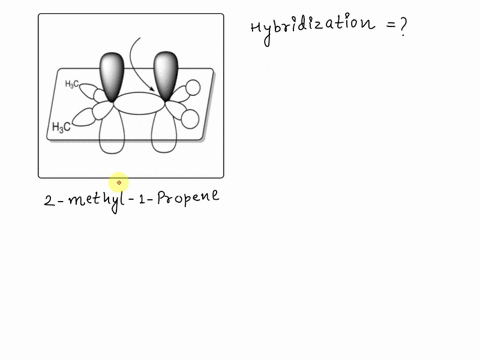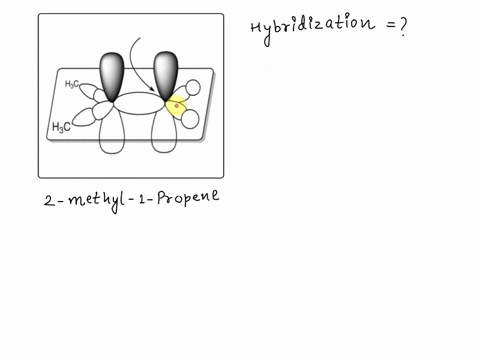
SOLVED: Texts: A picture of 2-methyl-1-propene with the molecular orbitals is shown. Identify the hybridization of the indicated carbon. A) sp B) sp2 C) sp D) sp3 E) sp2
How to draw a line-bond structure for propene, CH3CH≡CH2? What is the hybridization of the orbitals on each carbon - Quora