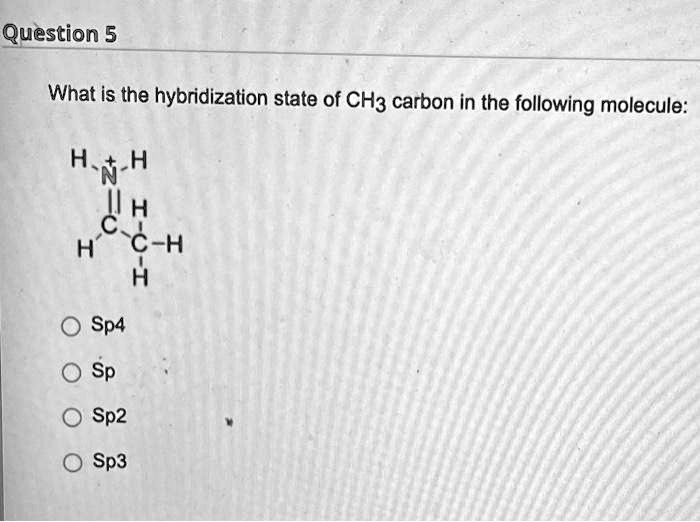
Hybridization of central atoms in the moleculesN(CH3)3 and N(SIH3) respectively are(1) sp2 and sp2(2) sp3 and sp3(3) sp2 and sp34) sp3 and sp2

CH3+and CH3- are two highly reactive carbon species. What is the predicted hybridization and geometry around each carbon atom? | Homework.Study.com

Doubt: What is the type of hybridisation of each carbon in the following compounds? (CH3)2CO , HCONH2 Chapter: Organic Chemistry - Some Basic Principles And Techniques - Subject: Chemistry - Course: NEET
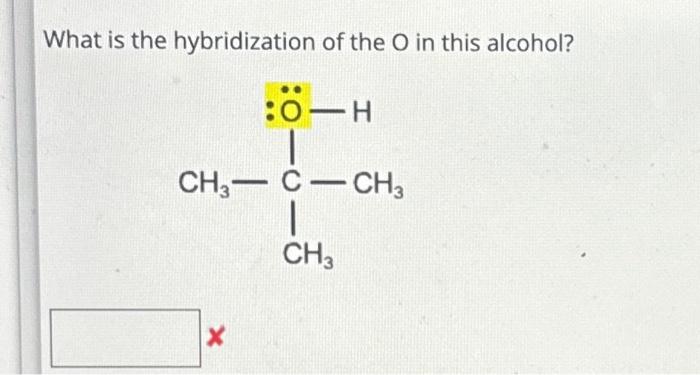
10.What is the hybridization of Oxygen molecule in the following : 1. CH3 O CH=CH2 2. CH3COCH3 [Propanone]
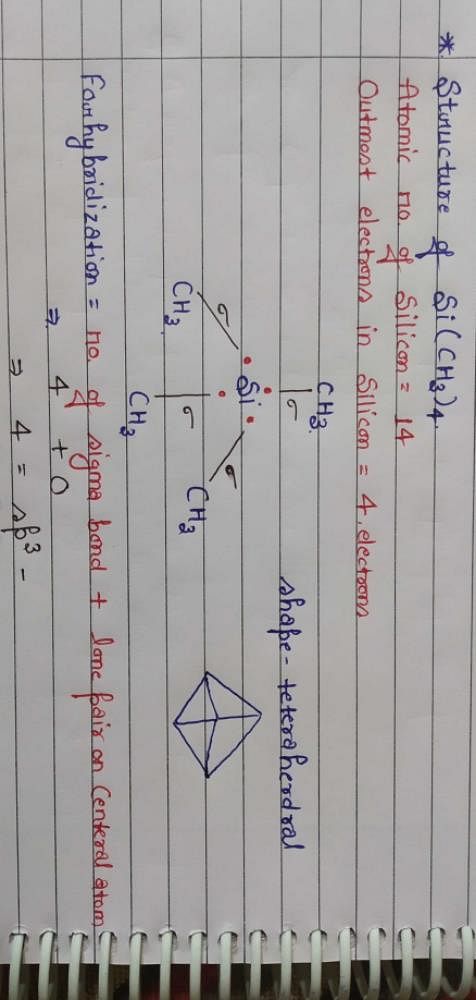
The structure and hybridisation of Si(CH3)4 isa)bent, spb)trigonal, sp2c)octahedral, sp3dd)tetrahedral, sp3Correct answer is option 'D'. Can you explain this answer? - EduRev NEET Question

Hybridization of C2 and C3 of H3C andndash; CH = C = CH andndash; CH3 area)Sp, Sp3b)Sp2, Spc)Sp2, Sp2d)Sp, SpCorrect answer is option 'B'. Can you explain this answer? - EduRev JEE

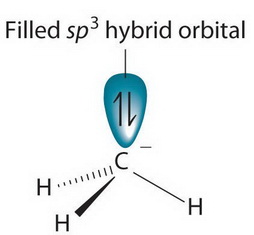
What is the hybridization of the carbon atom in each of the following CH_3^+, CH_3^-, CH_3? | Homework.Study.com