
In Cu-ammonia complex, the state of hybridisation of { Cu }^{ 2+ } is:{ sp }^{ 3 }{ d }^{ 3 }sds{ p }^{ 2 }{ sp }^{ 2 }f
Explain geometry of [Cu(NH3)4]^+2 on basis of hybridization. - Sarthaks eConnect | Largest Online Education Community

When crystals of CuS{ O }_{ 4 }cdot 4N{ H }_{ 3 } are dissolved in water, there is hardly any evidence the presence of Cu^{2+} ions or ammonia molecules. A new
Write down the hybridisation and magnetic character of the following complexes. (i) [Cu(CN)4]^2- (ii) [Cr(NH3)6]^3+ - Sarthaks eConnect | Largest Online Education Community
![SOLVED: Which is the correct statement(s)? A. [Ag(NH3)2]+ is linear with sp hybridized Ag+ ions. B. NiCl4^2-, CrO4^2-, and MnO4^- have tetrahedral geometry. C. [Cu(NH3)4]^2+, [Pt(NH3)4]^2+, and [Ni(CN)4]^2- have dsp^2 hybridization of SOLVED: Which is the correct statement(s)? A. [Ag(NH3)2]+ is linear with sp hybridized Ag+ ions. B. NiCl4^2-, CrO4^2-, and MnO4^- have tetrahedral geometry. C. [Cu(NH3)4]^2+, [Pt(NH3)4]^2+, and [Ni(CN)4]^2- have dsp^2 hybridization of](https://cdn.numerade.com/project-universal/previews/1561ff27-3e90-4193-9ac2-8cbafea01b2c.gif)
SOLVED: Which is the correct statement(s)? A. [Ag(NH3)2]+ is linear with sp hybridized Ag+ ions. B. NiCl4^2-, CrO4^2-, and MnO4^- have tetrahedral geometry. C. [Cu(NH3)4]^2+, [Pt(NH3)4]^2+, and [Ni(CN)4]^2- have dsp^2 hybridization of
![Cu(NH3)4]2+ এর সংকরায়ন। Hybridization of [Cu(Nh3)4]2+। @N00R007 #chemistry #hybridization - YouTube Cu(NH3)4]2+ এর সংকরায়ন। Hybridization of [Cu(Nh3)4]2+। @N00R007 #chemistry #hybridization - YouTube](https://i.ytimg.com/vi/z0R8tyAjAvY/maxresdefault.jpg)
Cu(NH3)4]2+ এর সংকরায়ন। Hybridization of [Cu(Nh3)4]2+। @N00R007 #chemistry #hybridization - YouTube
![OneClass: Cu in [Cu(CN)4]2- and [Cu(NH3)4]2+ has d9 electron configuration ,so the geometry should be... OneClass: Cu in [Cu(CN)4]2- and [Cu(NH3)4]2+ has d9 electron configuration ,so the geometry should be...](https://prealliance-textbook-qa.oneclass.com/qa_images/homework_help/question/qa_images/130/13010410.webp)
OneClass: Cu in [Cu(CN)4]2- and [Cu(NH3)4]2+ has d9 electron configuration ,so the geometry should be...
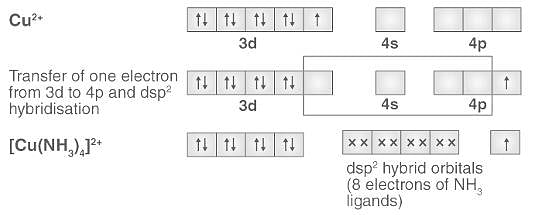
Cuprammonium ion has ______ shape.a)Octahedralb)Tetrahedralc)Trigonald)Square planarCorrect answer is option 'D'. Can you explain this answer? - EduRev Chemistry Question

Explain the Geometry of Cu(Nh3)4 2+ on the Basis of Hybridisation At. No. Cu = 29 - Chemistry | Shaalaa.com
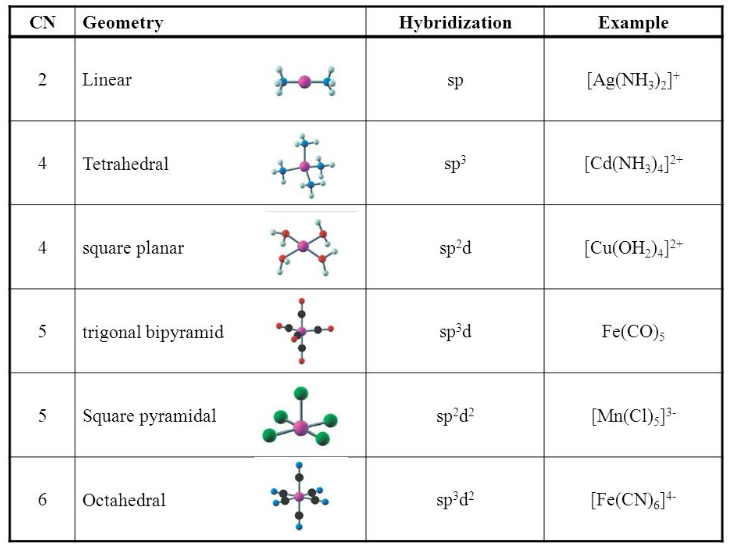

![Explain the geometry of [Cr(NH3)6]3+ ion by valence bond the Explain the geometry of [Cr(NH3)6]3+ ion by valence bond the](https://www.zigya.com/application/uploads/images/chen12070390_5714b9d19ba87.png)

![Explain the geometry of [Cu(NH(3))(4)]^(2+) on the basis of hybridisa Explain the geometry of [Cu(NH(3))(4)]^(2+) on the basis of hybridisa](https://d10lpgp6xz60nq.cloudfront.net/physics_images/GRK_10Y_SP_HSC_CHE_JLY_18_E02_001_S01.png)

![Hybridization and magnetic moment of `[Cu(NH_(3))_(4)]^(2+)` and `[Mn(CN)_(6)^(3-)` ions - YouTube Hybridization and magnetic moment of `[Cu(NH_(3))_(4)]^(2+)` and `[Mn(CN)_(6)^(3-)` ions - YouTube](https://i.ytimg.com/vi/owlgbfjVOzc/maxresdefault.jpg?sqp=-oaymwEmCIAKENAF8quKqQMa8AEB-AH-CYAC0AWKAgwIABABGGUgZShlMA8=&rs=AOn4CLBv6TqxUZtnijDOJn7QLMuUGig2-A)
![What is hybridization of [Cu (NH3)4 ]2+,[PtCl4]2-||Exception in coordination compound|| - YouTube What is hybridization of [Cu (NH3)4 ]2+,[PtCl4]2-||Exception in coordination compound|| - YouTube](https://i.ytimg.com/vi/4uNg5AAJJkA/sddefault.jpg)
![Cu(NH(3))(4)]^(2+) is a square planar complex.The number of unpaired Cu(NH(3))(4)]^(2+) is a square planar complex.The number of unpaired](https://static.doubtnut.com/ss/web-overlay-thumb/761702.webp)
![Hybridization in [Cu(NH3)4]2+ ion - YouTube Hybridization in [Cu(NH3)4]2+ ion - YouTube](https://i.ytimg.com/vi/mY8bvkr94QQ/sddefault.jpg)