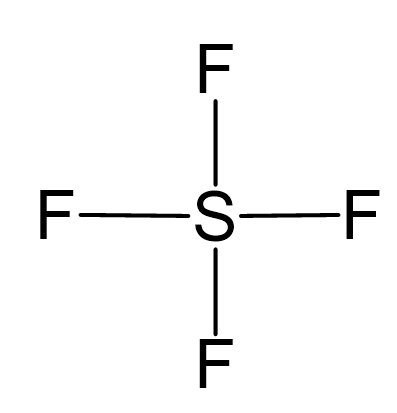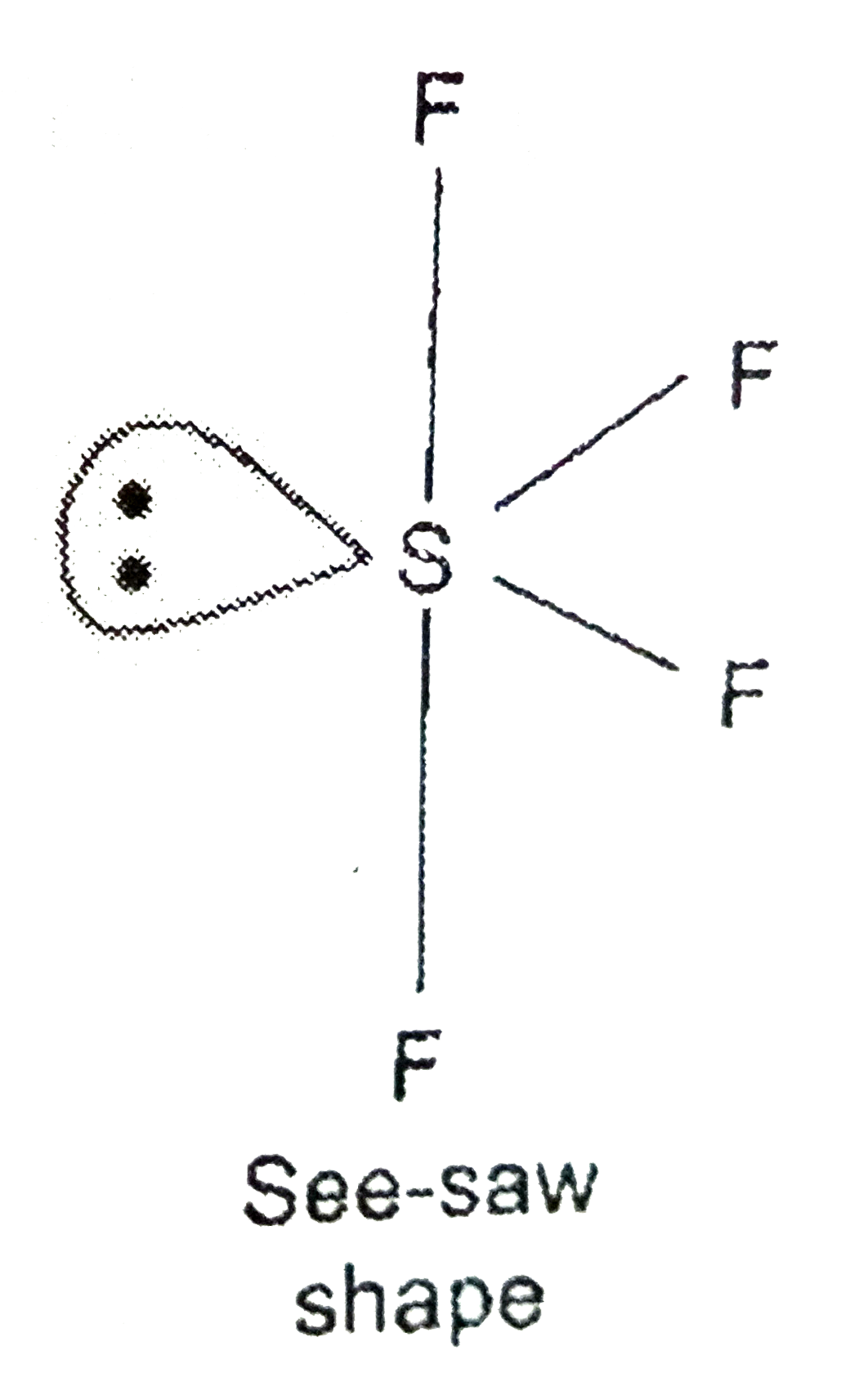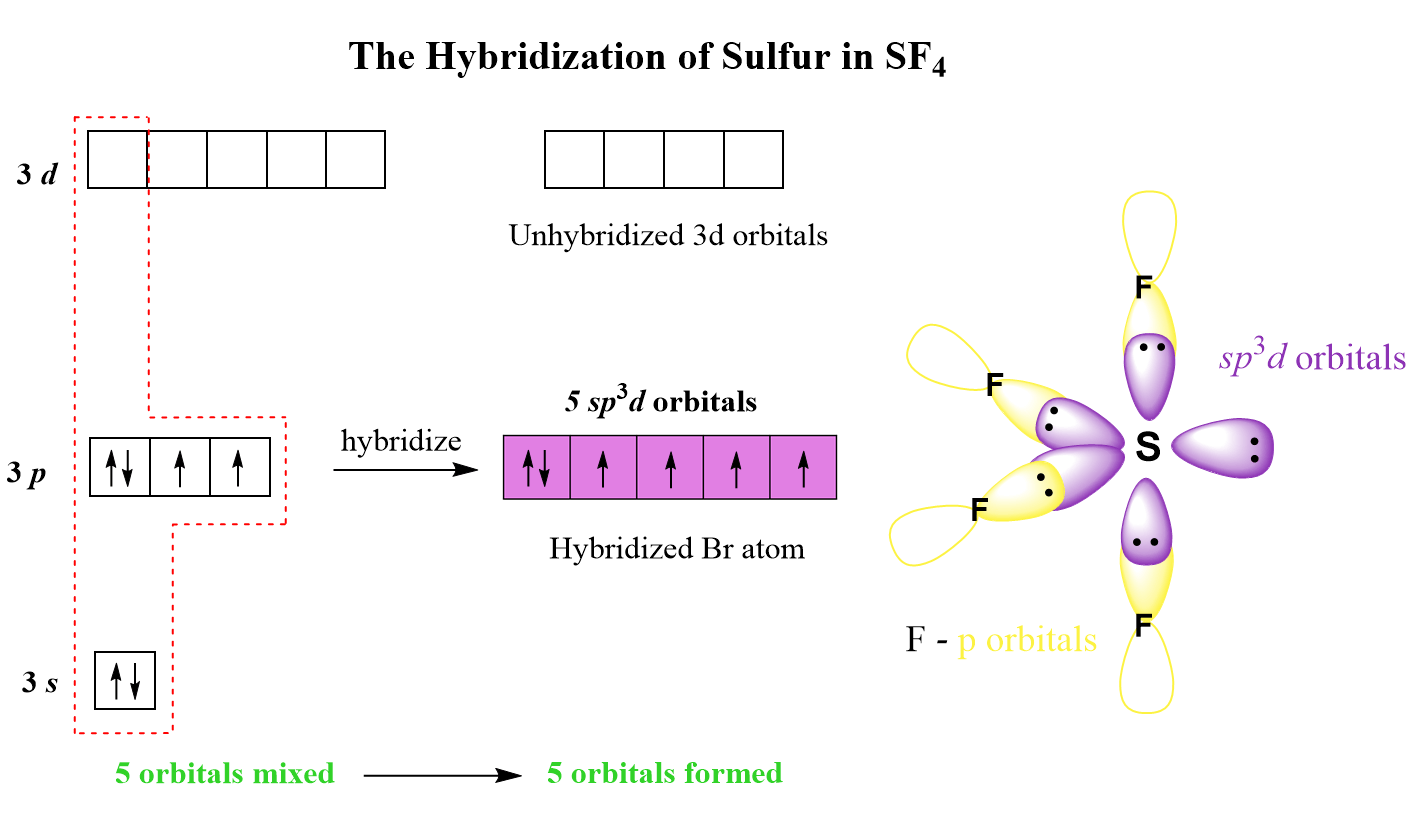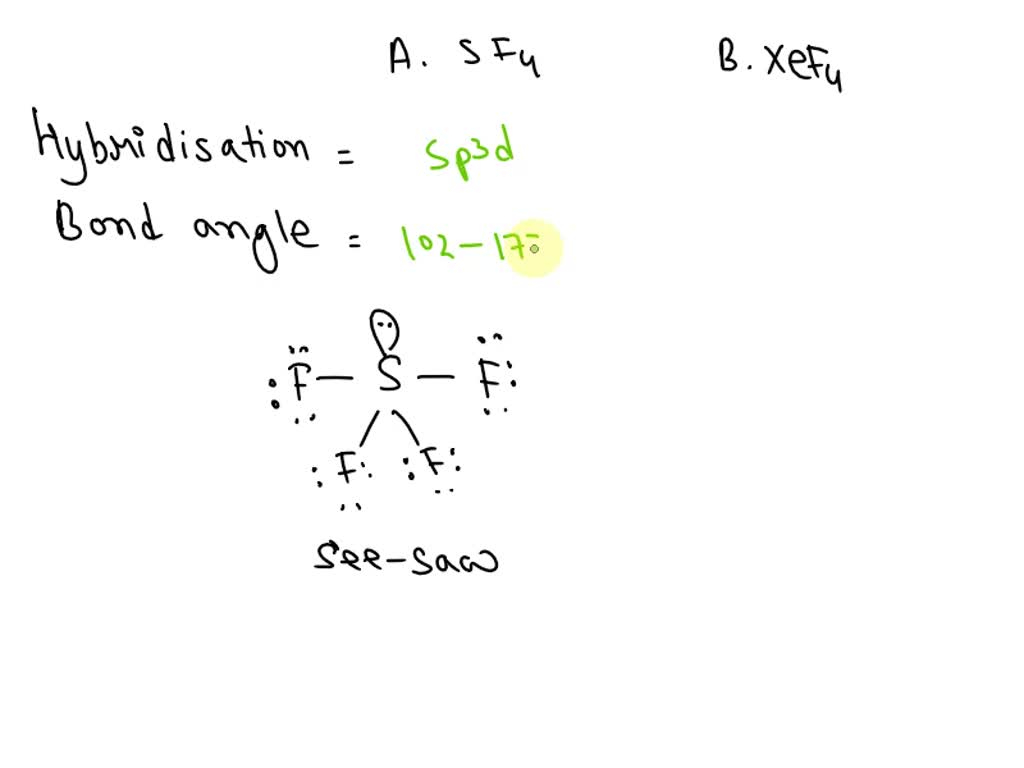
SOLVED: A. What is the hybridization of the central atom in SF4 Hybridization What are the approximate bond angles in this substance Bond angles What is the hybridization of the central atom

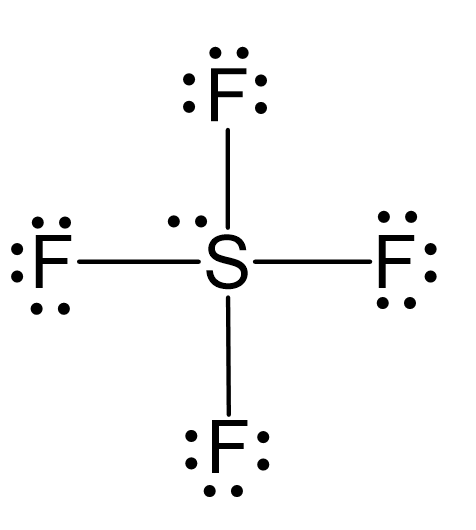
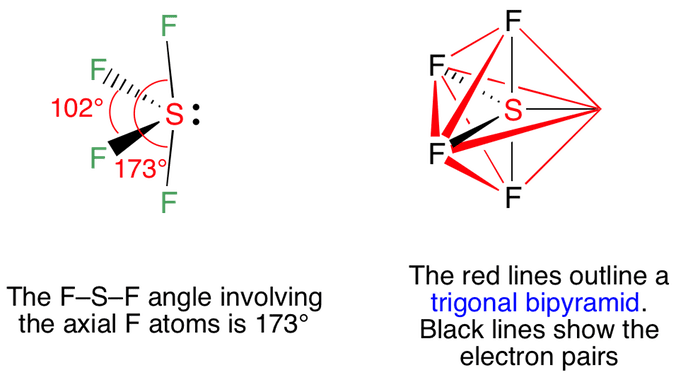
Draw the Lewis dot structure for SF4 and provide the following information. a. number of bond pairs b. number of lone pairs c. molecular geometry d. hybridization of the central atom

hybridisation of SF4 in this formula Hybridization=1/2(valency electron in central atom+no. Of atom attached - Brainly.in

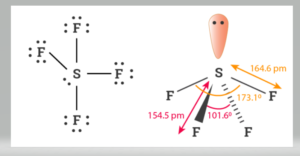
Welcome to Chem Zipper.com......: How to compare equatorial bond angle (F-S-F) and S-F bond length of SOF4 and SF4 molecules ?