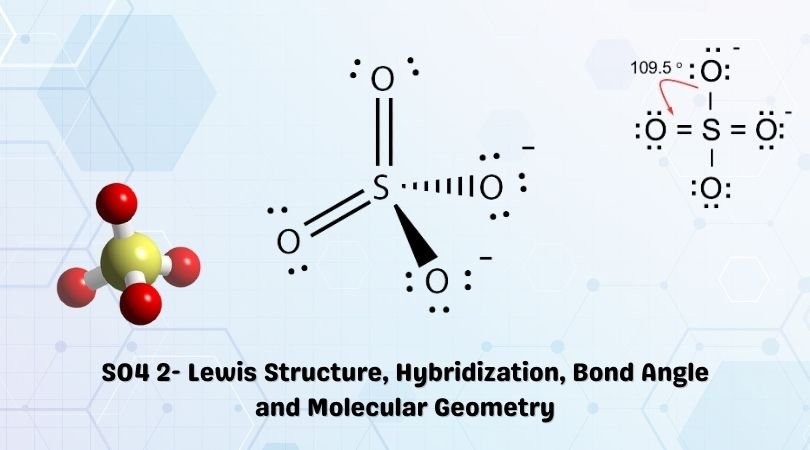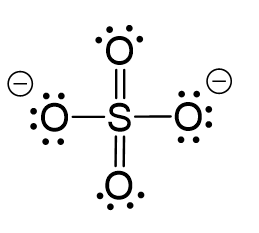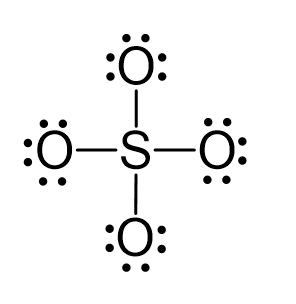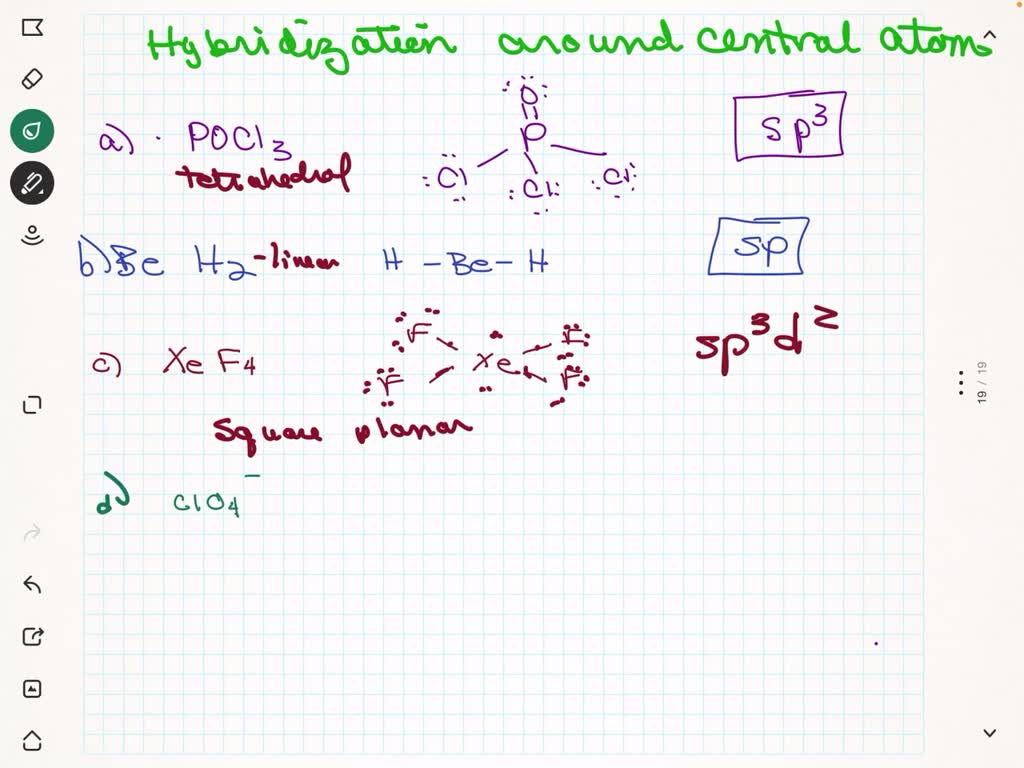
SOLVED: Give the expected hybridization of the central atom for the following molecules or ions. sp sp2 sp3 sp3d sp3d2 (a) POCl3 (b) BeH2 (c) XeF4 (d) ClO4- (e) BH3 (f) SO42-
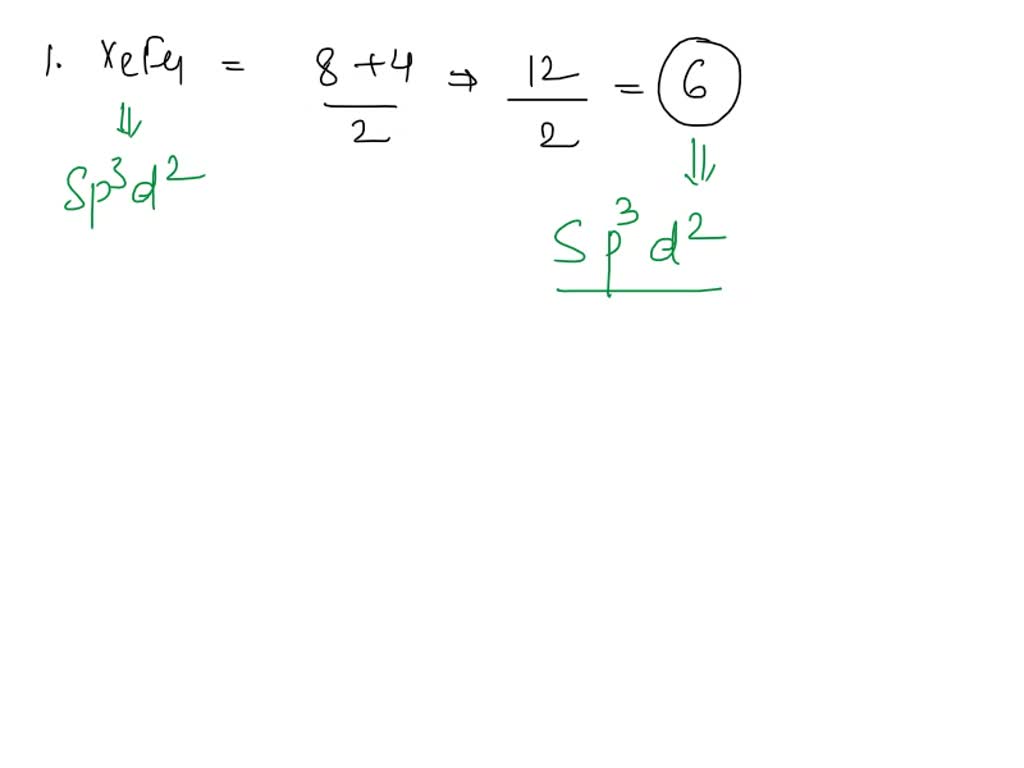
SOLVED: Give the expected hybridization of the central atom for the following molecules or ions: 1. XeF4 2. PH3 3. SO4^2- 4. BH3 5. PO3^3- 6. ClO2- 7. SO4^2-